RESPIRATORY VIRUS INFORMATION
The Centers for Disease Control and Prevention (CDC) has issued Respiratory Virus Guidance which has been adopted by the Connecticut Department of Public Health (CT DPH).
CDC’s guidance provides practical recommendations and information to help people lower health risks posed by a range of common respiratory viral illnesses, including COVID-19, Influenza (flu) and Respiratory Syncytial Virus (RSV).
| Respiratory Virus Guidance | Preventing Spread | CT DPH Press Release |
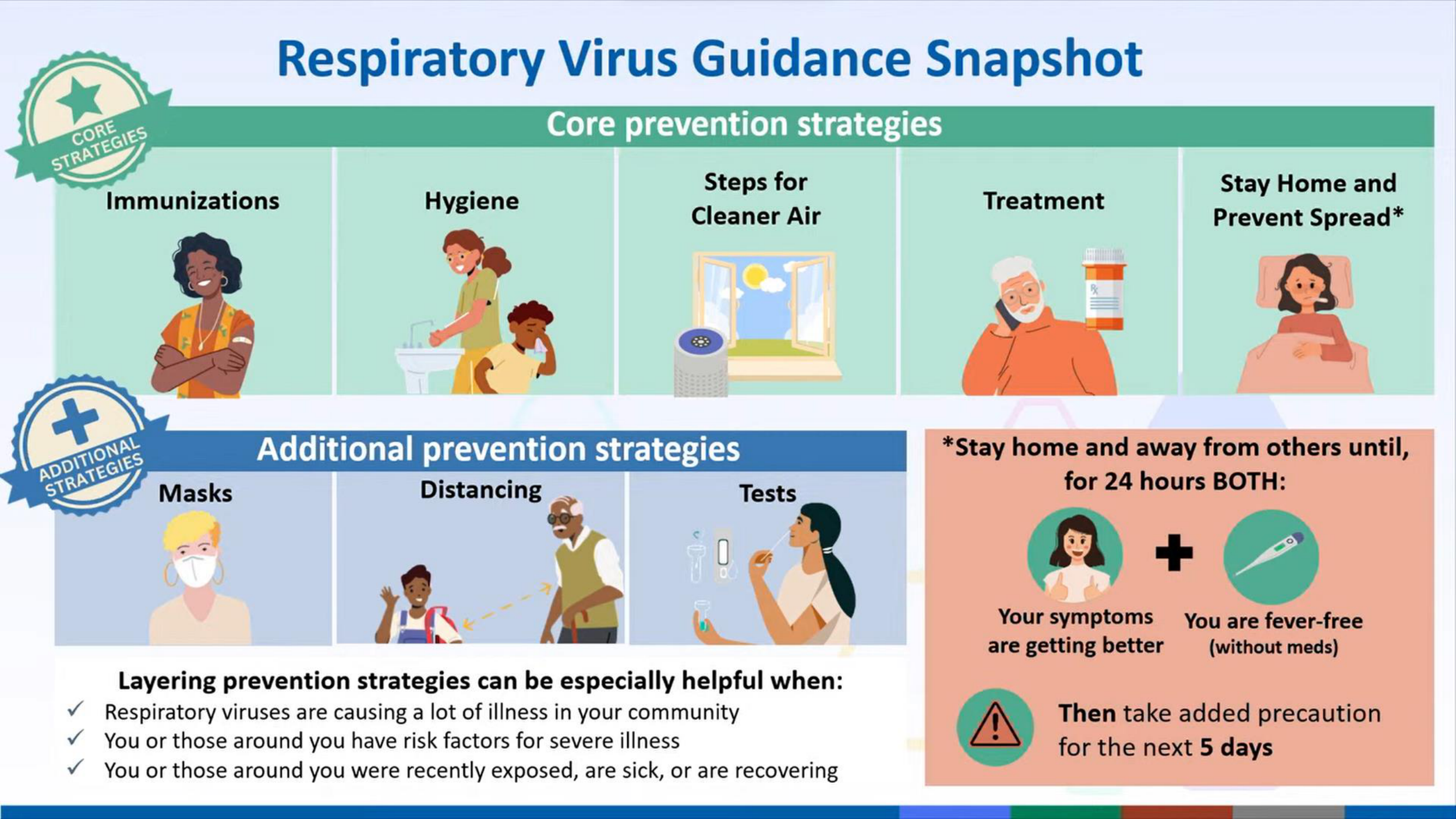
RESPIRATORY VIRUS GUIDANCE SNAPSHOT
Core Prevention Strategies:
ADDITIONAL PREVENTION STRATEGIES
WHEN YOU MAY HAVE A RESPIRATORY VIRUS...
Stay home and away from others (including people you live with who are not sick) if you have respiratory virus symptoms that aren't better explained by another cause. These symptoms can include fever, chills, fatigue, cough, runny nose, and headache, among others.*
|
You can go back to your normal activities when, for at least 24 hours, both are true:
|
When you go back to your normal activities: Take added precaution over the next 5 days, such as taking additional steps for cleaner air, hygiene, masks, physical distancing, and/or testing when you will be around other people indoors.
|
Below is a chart showing confirmed respiratory illness in Meriden for the 2023/24 season. This chart will not be updated after March 31, 2024.
